Streamline Clinical Trials with EDC Software Solutions
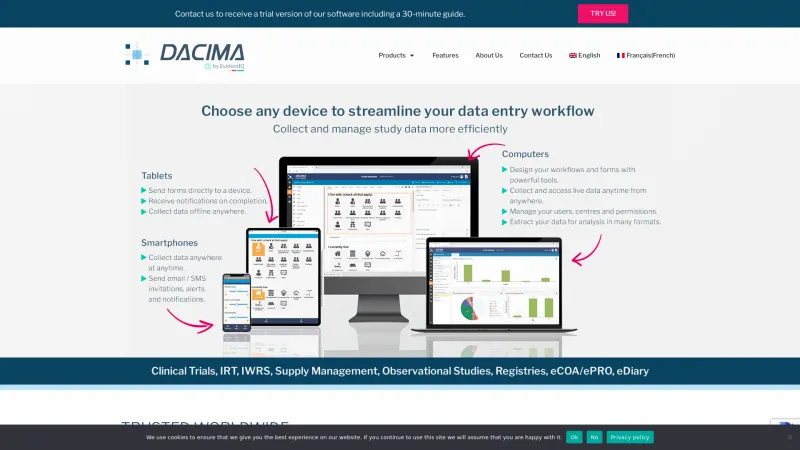
Electronic Data Capture (EDC) software is designed to streamline the collection, management, and analysis of clinical trial data. This software addresses the challenges of traditional data collection methods, such as paper-based forms, which can be prone to errors and inefficiencies. By digitizing the data capture process, EDC software enhances accuracy, reduces data entry time, and facilitates real-time data access. Read more
Key features of EDC software include customizable electronic case report forms (eCRFs), automated data validation, audit trails for compliance, and integration capabilities with other clinical systems. These tools not only improve data integrity but also accelerate the overall clinical trial process, enabling faster decision-making and reporting.
EDC software is best suited for clinical research organizations, pharmaceutical companies, and academic institutions involved in clinical trials. It is particularly beneficial for researchers and data managers who require a reliable and efficient way to collect and analyze data while ensuring compliance with regulatory standards.