Streamline Your Clinical Trials with DM365's MainEDC eClinical Solution
Clinical Trial Management SoftwareDiscover DM365's MainEDC platform, a cutting-edge eClinical solution that streamlines clinical trials, ensuring compliance and efficiency. Experience a free trial today!
About MainEDC
As a professional in the field of clinical research, I am consistently on the lookout for innovative solutions that can streamline processes and enhance data management. DM365's MainEDC platform stands out as a remarkable tool that addresses the complexities of clinical trials with impressive efficiency and reliability.
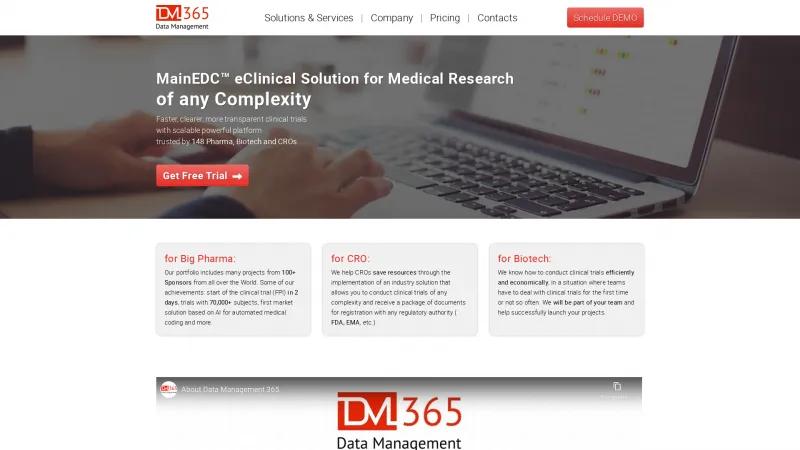
The MainEDC™ eClinical Solution is designed to cater to the diverse needs of Pharma, Biotech, and CROs, making it a versatile choice for organizations of all sizes. The platform's ability to facilitate faster clinical trials—achieving First Patient In (FPI) in as little as 2 days—is a game-changer in an industry where time is of the essence. The statistics provided, such as managing trials with over 70,000 subjects and the implementation of AI for automated medical coding, underscore the platform's capability and innovation.
One of the standout features of MainEDC is its commitment to compliance with industry standards and regulations, including ICH GCP E6(R2), HIPAA, and GDPR. This level of adherence not only ensures the integrity of the data but also instills confidence in users regarding the security and privacy of their information.
Moreover, the platform's user-friendly interface, which includes tools for easy eCRF building and advanced visual edit-checks, significantly reduces the learning curve for new users. The promise of ongoing support and analytics further enhances the user experience, making it clear that DM365 is dedicated to the success of its clients.
The transparency and collaborative approach DM365 offers through its Technology Transfer service is particularly commendable. By sharing expertise and best practices, they empower organizations to navigate their first projects with confidence, ensuring a smooth transition to using the MainEDC platform.
DM365's MainEDC platform is a robust solution that not only meets the demands of modern clinical trials but also sets a new standard for efficiency and quality in data management. I highly recommend exploring this platform for any organization looking to enhance their clinical research capabilities. The opportunity for a free trial is an excellent way to experience the benefits firsthand, and I encourage interested parties to take advantage of this offer.
Electronic Data Capture Features
- Audit Trail
- CRF Tracking
- Data Entry
- Data Verification
- Distributed Capture
- Document Imaging
- Forms Management
- Remote Capture
- Study Management
Clinical Trial Management Features
- 21 CFR Part 11 Compliance
- Document Management
- Electronic Data Capture
- HIPAA Compliant
- Monitoring
- Patient Database
- Recruiting Management
- Scheduling
- Study Planning
Leave a review
User Reviews of MainEDC
No reviews yet.