TT Science eCRF System: Innovative Clinical Trial Solution for Enhanced Workflow and Data Integrity
Clinical Trial Management SoftwareDiscover TT Science's eCRF system for clinical trials, featuring seamless hospital integration, real-time data validation, and advanced analytics for efficient research management.
About Transition Technologies eCRF
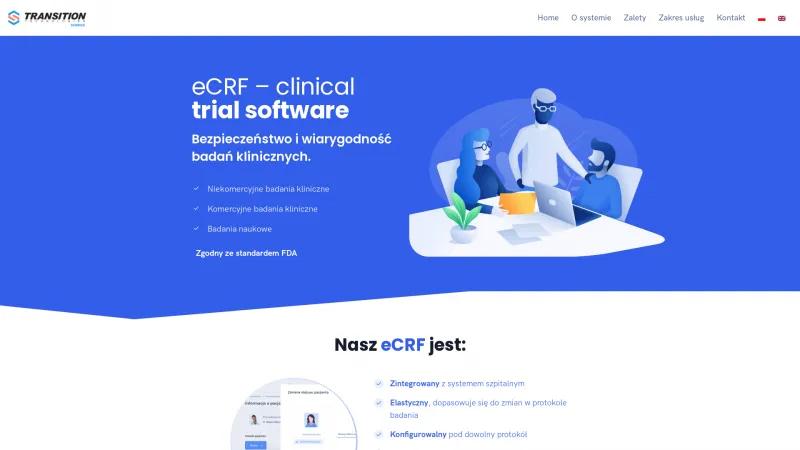
TT Science's eCRF system is a remarkable tool for clinical trials, showcasing a blend of innovation and user-centric design. The platform stands out for its commitment to safety and reliability in clinical research, whether for commercial or non-commercial studies.
One of the most impressive features is its integration with hospital systems, which enhances workflow efficiency. The eCRF is not only flexible and customizable to various protocols but also quick to implement, making it an ideal choice for researchers who need to adapt to changing requirements swiftly.
The system's ability to manage multiple clinical trials from a single interface is a game-changer. It provides a comprehensive view of patient statuses and facilitates effective management of trial processes, including randomization and product tracking. The automatic generation of unique patient codes and real-time data validation significantly reduces the risk of errors, ensuring high-quality data collection.
Moreover, TT Science's eCRF offers advanced functionalities such as anomaly detection and interactive dashboards, which empower researchers to monitor trials proactively. The integration of statistical reporting and machine learning algorithms for hypothesis generation further enhances its utility, making it a forward-thinking solution in the realm of clinical research.
TT Science's eCRF system is a powerful, intuitive, and adaptable tool that meets the rigorous demands of clinical trials while ensuring compliance with FDA standards. Its comprehensive features and user-friendly interface make it an invaluable asset for any research team.
Leave a review
User Reviews of Transition Technologies eCRF
No reviews yet.