Ketryx: Revolutionizing FDA, EU MDR, and ISO Compliance for Medical Software Development
Software Bill of Materials (SBOM) ToolsRevolutionize your medical software development with Ketryx. Achieve FDA, EU MDR, and ISO compliance effortlessly while enhancing productivity and automation.
About Ketryx
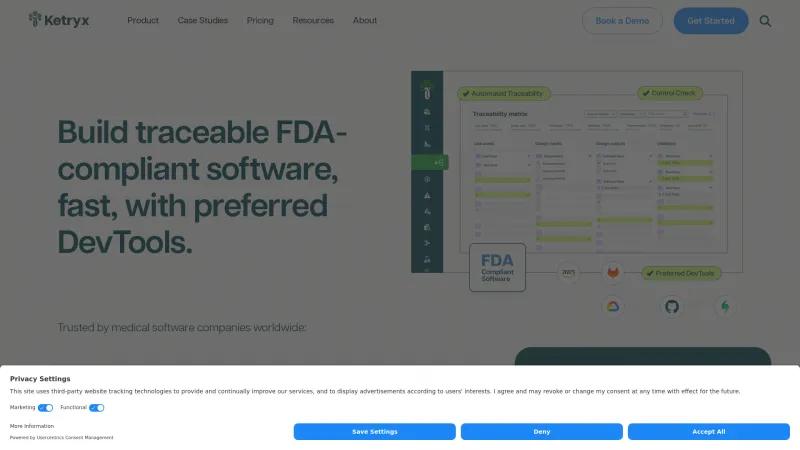
Ketryx is a game-changer in the realm of FDA, EU MDR, and ISO compliance software, particularly for those involved in the development of regulated medical applications. Their Connected Lifecycle Management platform stands out for its ability to seamlessly integrate with popular development tools like Jira, GitHub, and AWS, allowing teams to maintain their preferred workflows while ensuring compliance.
One of the most impressive features of Ketryx is its focus on automation. The platform not only generates submission-ready design history files (DHF) but also creates real-time traceability matrices that connect requirements, risks, code, and tests. This level of automation significantly reduces the complexity and time associated with software development in the medical field, enabling teams to release safer products faster.
Moreover, Ketryx's commitment to quality management systems (QMS) is evident in its ability to enforce SOPs and ensure that teams remain audit-ready throughout the software development lifecycle (SDLC). This proactive approach to risk management and documentation is crucial for organizations striving to meet stringent regulatory standards.
The testimonials from industry leaders underscore Ketryx's effectiveness. Users have praised its bi-directional synchronization capabilities, which enhance productivity and streamline processes. The platform's ability to support agile development while maintaining compliance is a significant advantage for R&D teams.
Ketryx is an essential tool for any organization involved in medical software development. Its innovative features, robust integrations, and focus on compliance make it a top choice for quality professionals and R&D leaders alike. If you're looking to accelerate your development process while ensuring regulatory adherence, Ketryx is undoubtedly worth considering.
Leave a review
User Reviews of Ketryx
No reviews yet.